
On Saturday morning as I was heading out for a run at the Chattahoochee River, I found my car in the condition you see in the photo above. Let’s ignore the reason I was parked half in and half out of the garage and focus on the frost instead. As you can see, the frost appeared only on the back part of the car. The part of the car that was under the roof had no frost at all.
The physics of this phenomenon is really interesting so let’s examine it, then we’ll see how the principle involved affects what happens in buildings, specifically the comfort felt—or not felt—by occupants.
Do frost and dew fall out of the air?
I didn’t get a photo of the top of the roof over my garage, but it also was covered with frost. Frost appearing on the highest surfaces makes it look like water vapor just falls out of the air and hits the first surface it finds. According to meteorologist Paul Knight, quoted in an excellent 1985 Chicago Tribune article on this topic, that was the common belief up until the beginning of the twentieth century.
But it’s wrong. The air around a house, above a house, and in the open garage or carport all have similar concentrations of water vapor. But there’s nothing that would cause water vapor molecules to drop out of the air at a certain temperature and land on the first surface they find below where they began falling.
If that were true, frost would have formed on the part of my car under the roof. It just would have been a thinner layer because of less water vapor in that smaller space between the soffit and the car roof. But no matter how closely you look, there’s no frost on that part of the car.
Understanding thermal radiation
Since gravity doesn’t explain the frost-free surface under the roof, what does? The answer is in an article I wrote nine years ago, Naked People Need Building Science. In that article I wrote:
Every object radiates heat. The amount of radiant heat it gives off depends on its temperature (to the 4th power!), surface area, and emissivity.
Anything that has a temperature will produce thermal radiation (also called radiant heat) by the jiggling of molecules within. That thermal radiation removes heat from the object. But the object is also receiving radiant heat from all the surrounding objects. The image below is from my Naked People article and it illustrates the imbalance in radiant heat transfer in that case. The single pane window at 35°F doesn’t radiate nearly as much heat back to the naked man as the naked man radiates to it. So there’s a net cooling, at least in that direction.
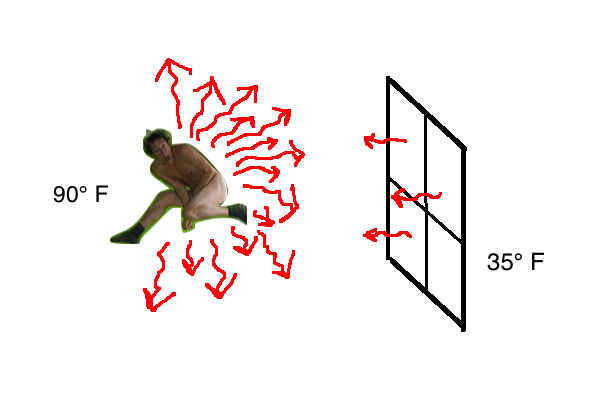
The same thing happened with my car Friday night. The difference between the frosty back of the car and the frost-free front of the car was the radiant heat balance. The back of the car was exposed to the night sky; the front of the car to the soffit and garage ceiling, which is much warmer than the night sky.
The night sky turns out to be quite cold. It can absorb a lot of thermal radiation without sending much back. The result is that a terrestrial object—the back of my car in this case—under a clear night sky will get colder. That same object under a roof—like the front of my car—will have a more balanced transfer of radiant heat and thus will stay warmer.
There’s your answer. The back of my car got colder than the front of the car because it radiated away a lot of heat to the night sky without getting much in return. And that temperature decrease put the surface of that part of the car below the frost point (the below-freezing equivalent of dew point).
The implications for comfort
My Naked People Need Building Science article discusses the effect on comfort of being naked in front of a cold window. As with the car getting colder when exposed to the night sky, a person exposed to a cold surface in a building will feel colder than when they’re surrounded by warmer surfaces.
My friend Robert Bean up in Calgary, Alberta deals with this issue all the time. And not just because he lives in such a ruthlessly cold environment. He’s also a member of ASHRAE’s committee on thermal comfort. A lot of people think of air temperature and humidity and sometimes air movement when asked what factors affect thermal comfort, but the big one that doesn’t get nearly as much attention as it should is the one related to the temperatures of the surrounding surfaces. It’s called mean radiant temperature and factors in the temperatures of all the stuff around you. The warmer they are, the warmer you are.
So think of that frost on the back of my car as you’re standing in front of a big single pane window on a cold night (with or without clothes, your choice). And the frost-free front of the car is you standing in front of well-insulated, air-sealed walls.
This same phenomenon applies also to cases where our buildings make us too warm. Bonus rooms are the most common culprit there, but it can happen in other places where you have high temperatures on one side of an inadequate building enclosure component.
And do you know why it’s called mean radiant temperature? This history of the term is not well known but it does help you understand it. It’s called mean radiant temperature because if you don’t pay attention to the surface temperatures in a building, they will be very mean to you. (OK, I’ll admit it. That history isn’t well known because I just made it up. But you should feel free to share it nonetheless.)
–Allison Bailes of Decatur, Georgia, is a speaker, writer, building science consultant, and the author of the Energy Vanguard Blog. You can follow him on Twitter at @EnergyVanguard. Photo and illustration courtesy of the author.
Weekly Newsletter
Get building science and energy efficiency advice, plus special offers, in your inbox.














8 Comments
"The night sky turns out to be quite cold. It can absorb a lot of thermal radiation without sending much back. "
This may seem a minor quibble, but shouldn't we say the night sky (especially a clear one) can TRANSMIT a lot of thermal radiation? If it absorbed it, it would ultimately re-emit it in a diffuse manner, including back to the ground.
The original phrasing makes it sound like the night sky acts as a sort of sink. And while it surely does to some degree (obviously some energy is still absorbed) the trait that works to increase the cooling effect is the transmission.
This would clarify why a cloudy sky, which indeed absorbs a lot of radiation, offers a slower cooling mechanism—because it has more 'stuff' in it to absorb the energy and ultimately re-emit. The 'cleaner' the sky, the higher its value of transmissivity, and the greater the cooling effect.
Unless I'm missing something, like: is the fact the the sky and its material is 'cold' (a relative term) mean it actually can simply absorb a lot of energy without emitting as much back? In this case, a 'clean' sky simply means a cold sky. Hmm. That actually sounds reasonable. I've confused myself a bit. oh boy.
edit: i'm going to stick with my original assertion for the time being, for the reason that a sky composed of fewer particles (a clear, clean sky) would allow increased cooling due to transmission. As we approach vacuum, it becomes harder to assign a temperature to it anyways. In such a case, as we approach said vacuum, we're relying completely on transmission.
If this is a detached, unheated garage the temperature difference between it and the surrounding air/night sky may not be that much different. However, there is a huge difference between the amount of radiated energy emitted from a solid versus a gas at the same temperature. The energy emitted should be proportional to the number of atoms in each, which will be orders of magnitude higher in the solid garage materials versus the gaseous air outside.
Nice Dan. I brought up in another thread here that the reason an object can radiatively cool beyond 'ambient' (air) temperature has to do with the emissivity constant, which sounds similar to your statement regarding radiation rates of solids vs gases). I believe there's more to it than sheer number of atoms—such as the molecular boundary structure— which is why low-e coatings (which are solid) can exist.
One maybe could argue that the 'effective' temperature difference between the air and the solid garage—when regard is given to radiation measurements— IS lower for the air, since more energy is (as I pointed out above) transmitted. In other words, if you pointed an IR thermometer at the sky, you wouldn't read the temperature of the immediately surrounding air.
It turns out its really hard to accurately read the temperature of a gas without compensating for radiation exchange. That's why we need to put standard thermometers in the shade if we don't want the sun's direct radiation to jack up the ambient air temperature measurement, and likewise, why an old school thermometer sitting on the top of that same car might read lower than the ambient air via it's own radiative cooling. This is what 'effective' means, though you are correct that an accurate ambient air temperature reading would read similar to such objects as the underside of the overhang.
Adding to what already has been said, the phenomenon shown in the photo is far less likely to occur without still air and a cloudless night. Without air movement to mix up the air, a thin film of supercooled air will form right next to the surface facing directly up and radiating its heat away to the cosmos. That film temperature will be substantially lower than that of the bulk air above it.
The opposite effect is seen on a clear but bitterly cold day after a snowstorm, even with some breeze, and a paved driveway has been shoveled off. The sun shining on the driveway will warm up a thin boundary layer of air above any small patches of bare pavement, melting snow at the edges, and those bare patches grow in size.
Making good air temperature measurements usually requires having placing the thermocouple or thermometer bulb in the shade and inside a radiation shield covered with a highly polished low-emissivity surface.
You all might want to read this primer on sky temperature:
https://www.designingbuildings.co.uk/wiki/Sky_temperature#:~:text=
On clear nights the sky temperature is much MUCH lower than the air temperature, and often below the dew point temperature of the air. Surfaces that are facing open sky will often drop to the dew point temperature of the proximate air and moisture will deposit on the surface. The surface temperatures don't drop more than a miniscule amount below the dew point temperature of the air due to the heat of vaporization (or in the case of frost, the heat of vaporization AND the heat of fusion) of that condensing moisture.
The air itself is fairly translucent to most of the deep infra-red spectrum. Air films next to the surface can insulate against warmer air films above it, but does effectively nothing to block the radiant loss.
Where there is an overhanging roof or tree blocking the radiant path to the sky, the surface temperature of the surface with the blocked path will stay close to the ambient air temperature.
> "On clear nights the sky temperature is much MUCH lower than the air temperature"
Since I can't see any other comments that this would be in response to, I assume it's Dan's (#2), and my subsequent (#3), discussing of air temperature. Your point is taken that the 'night sky' and the immediately adjacent air are not the same temperature. That was the point in discussing effective temperature in regards to radiation, since it's ultimately being transmitted into space. The relevant temperatures are throughout all layers of atmosphere from ground to space.
We don't say it's solely and absolutely the temperature of space (top most layer) any more than we say its solely and absolutely some arbitrary point in the upper atmosphere (middle layer). There are particles throughout the medium acting as grey bodies and emitting radiation back towards the ground. The 'effective' night sky temperature calculations are not a temperature at a specifically defined point; they are an approximation of effective radiation output towards the ground given the sum of all contributing grey bodies in the stack-up; from ground to space. Although some approximations will use a true theoretical black body. See the following from http://www.ibpsa.org/proceedings/BS2017/BS2017_569.pdf:
"The concept of an effective or equivalent temperature is
introduced in the domain of building simulation to
describe the temperature of the atmosphere, even though
it contains several layers at different temperatures. This
temperature is termed Effective Sky Temperature (𝑇𝑠ky),
and it provides a practical approach to approximate the
infrared heat transfer from the atmosphere (“sky”). After
introducing the sky temperature, the amount of
longwave radiation emitting by the sky can be calculated
according to the Stefan-Boltzmann law if regarding the
sky as a black body.
After reading dan's comment again, and my post #3, I'm possibly seeing the concern for confusion.
To clarify my statement on the emissivity constant allowing greater cooling of an object than ambient:
The reason I say this is because if the ambient air were considered an equal emitter to the body in question (e.g. car roof), that ambient air would cool via the same principle in discussion at the same rate if all else were equal. (Simplified, ignoring the convective complexities of the air for a moment). If we treat a pocket of air as an emitting body itself, and that body emitted at the same rate (same constant) as the car roof, than that pocket of air would cool at the same rate. We would never see an object cool below ambient.
I had seen an emissivity value ascribed to air (and it was indeed lower than most solid objects) and this was the reason for my statement. I'm realizing that perhaps that ascribed value is also more of an 'effective' value and isn't truly applicable to a gas system, which is complex in terms of emissivity behavior.
This exact thing happens to me all the time, this morning even, when my windshield was wet and the back window had ice on it.
We park two cars in a single lane driveway in front of our two story townhouse. The windshields of both cars are pointed at the (relatively warm) house, and the back windows are pointed directly up at the sky.
Quite often in the morning the windshields will be dry and the back windows will be wet. Too bad I’m the only one ever out there... I have no one to share this amazing physics with!
Log in or create an account to post a comment.
Sign up Log in