
“My new water heater smells like rotten eggs!”
Since we started installing heat-pump water heaters a few years ago, we’ve gotten this call several times. The “rotten egg” odor, hydrogen sulfide (H2S) gas, seems to show up almost exclusively in rural homes on well-water systems. Heat-pump water heaters are becoming popular in this market; they offer a green and cost-effective alternative to electric resistance, oil, or propane. I was relieved to learn that heat pump technology isn’t the cause of the problem; under the right conditions, H2S smells can occur in almost any tank-style water heater.
The problem usually shows up days to weeks after we replace an older water heater. The odor wasn’t there before, and it’s present only on the hot water side of the system. In milder cases, it dissipates after the water runs a few minutes, but in severe cases, it can persist and spread throughout the house.
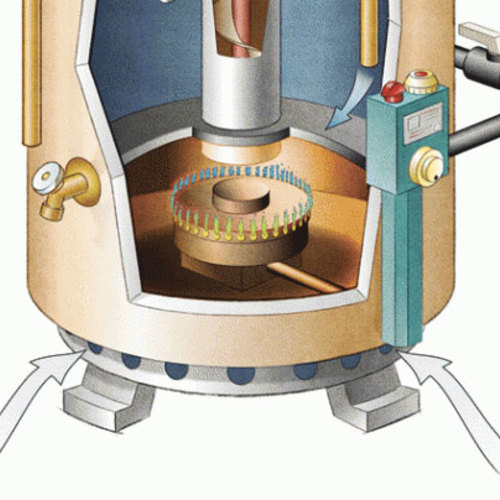
The levels of H2S found in tap water aren’t high enough to be a health concern, but they can impact quality of life. Understanding what causes H2S smells—and how to resolve them—requires understanding a bit about water heater chemistry and microbiology. In particular, it requires thinking about a corrosion protection device, the sacrificial anode, and how it can stimulate the growth of odor-causing bacteria.
Understanding sacrificial anodes
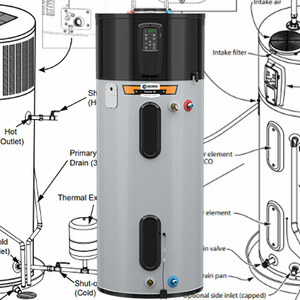
Most hot water tanks are made of steel lined with a layer of glass. Over time, tiny cracks develop in the glass lining, exposing the steel to water in the tank. In the absence of additional protection, the steel in the tank will oxidize, corrode, and eventually leak.
At the molecular level, “oxidation” means that iron atoms, the main component in the steel, are giving up electrons. When this happens,…
Weekly Newsletter
Get building science and energy efficiency advice, plus special offers, in your inbox.

This article is only available to GBA Prime Members
Sign up for a free trial and get instant access to this article as well as GBA’s complete library of premium articles and construction details.
Start Free TrialAlready a member? Log in













11 Comments
Great article, you have described exactly what I am seeing at our farm with hot water. Now I have a solution. Thank you, Jon
Thanks Doug. This issue has come up often enough in our projects that I thought others could benefit from our learning curve!
>Besides providing long-lasting and maintenance-free corrosion protection, they reduce the buildup of mineral scale, another major cause of water heater failure.
Do you have any more information on why this might be the case? My understanding is that mineral scale forms due to some equilibrium shift of calcium carbonate solubility at higher temperatures -- not something that I would think would be affected by the electrochemistry involved in corrosion protection.
I found this claim in a couple places. I think some of it may have to do with the extra magnesium ions from a standard anode, which can also form insoluble carbonate scale. If I find more info, I'll update.
This is great!
I have one more suggestion for how to use the powered anode kit. If you haven't yet put in a heat-pump water heater, because you are waiting for your old water heater to die first, you can get the kit, hook it up backwards, and accelerate the end of life of that obsolete equipment, given you an excuse to upgrade to a heat pump. (Just kidding.)
Jon,
Another great blog.
I've had some nightmare experiences trying to replace anodes though. On many electric resistance hot water heaters the anode is buried in spray foam and recessed enough you need a deep 7/8" socket - which most people don't have. They are often very difficult to remove. I've had two friends hold a tank still while I used a bar extension to try and break one free. Finally had to give up.
Thanks Malcolm! That's a good point, you will need deep sockets to get to the recessed anodes. One of my techs suggested that it can be easier to remove the anode when the tank is cool and the bushing has shrunk ever so slightly.
If the plan is to install a powered anode rod on a brand new unit would it make financial sense to opt for say a 6 year tank instead of a 10 year tank? In the past I felt like paying a bit more for a longer tank life made financial sense but in this case maybe I’d be mostly paying for a larger anode that I’d only be discarding? Any other benefits to the more expensive heaters?
With a heat-pump water heater the other differences are the efficiency (COP) of the heat pump and the longevity of the heat pump hardware.
Thanks! In my case I’m planning to go with a conventional unit. Any other benefits with those?
Heat-pump water heaters are a good solution here in NC when the water heater is in a garage or basement. In addition to using less energy, they will dehumidify the surrounding air and cool it. This further improves the efficiency if it is in an area where their is already a dehumidifier that runs often anyway. In heat-pump only mode, they will produce about 7 gallons per hour so the savings decline in high use scenarios where the resistive element is used more often. Although they claim to be quiet, I would be cautious about installing them near living space as the fan does make noise. You will need more headroom than a standard tank and condensate drain will need to considered. If replacing an existing 30 amp electric tank, the existing whip can be used (assuming it is code compliant).
Log in or become a member to post a comment.
Sign up Log in