Air has weight. Air can move things, like giant wind turbine blades. Air can also crush things. I first got to see the power of air in my introductory physics class in college. The professor, Dr. Jeffrey Trahan, showed the class an empty steel can just like the one in the photo below. He then did a little manipulation of the physical parameters. The result was that the can, made with a fairly heavy gauge of steel, got crushed right before our eyes. I did this demonstration in my own classes later on, but now there’s an easy way for anyone to do it. I call it the ol’ beer can pressure crunch.
Balanced and unbalanced pressures
When you hold out your hand, the weight of the air above it is about 200 lb. You don’t feel it, though, because that weight causes the air around your hand to exert pressure in all directions. That means the 200 lb. pushing down on your hand is matched by 200 lb. pushing up. Thus, you don’t have to do any work to hold up that air. It’s actually the air below your hand holding it up.
To see the effect of air pressure, we have to get it to push fully on one side of an object with a reduced pressure on the other side. That’s how Dr. Trahan crushed the steel can. And it’s how I crushed the aluminum beer can in the lead photo. Here’s video showing the beer can pressure crunch, with a short explanation at the beginning.
Psychrometric trickery
The way I (and Dr. Trahan) achieved a pressure difference between the inside and outside of the can was through a little psychrometric trick called condensation. I put a little bit of water in the can and and then set the can on a hot burner on my stove. Once the water got to a full boil and I saw steam escaping from the top, I screwed the cap on and removed it from the heat.
At first, the pressure inside the can is equal to the pressure outside, and nothing happens. The air inside, however, is different from the air outside. It has a much higher percentage of water vapor in it than the air outside. But that’s a temporary state.
As the can cools, so do the air and water vapor inside the can. The water vapor starts condensing on the inside of the can. As the water vapor leaves the air, the pressure inside the can decreases. That puts a pressure difference across the can, with the outside pressure getting getting more and more of an advantage. At a certain point, it crushes the can, as you saw in the video.
Other cool pressure tricks
You can see dozens, maybe hundreds, of videos of this demonstration on YouTube. Most of them do it with a pop-top can, which the demonstrator quickly removes from the heat and dunks upside down into a bowl of ice water. I like the slow approach better, and screw-top beer cans work perfectly!
I mentioned that my first experience with this demonstration was with a heavier gauge steel can, but it can go even further. Here’s a video showing the same thing happen to a 55 gallon drum. Yes, really!
This isn’t the only way crush a can with air pressure, of course. You could simply use a vacuum pump to pump the air out, and the same thing would happen.
Applications to building science
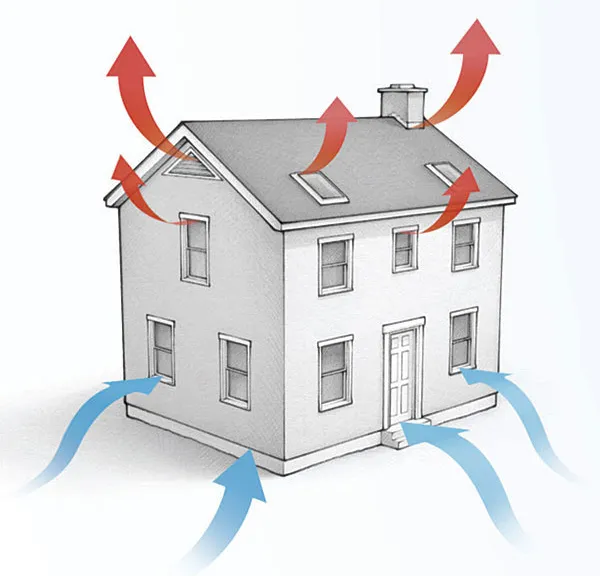
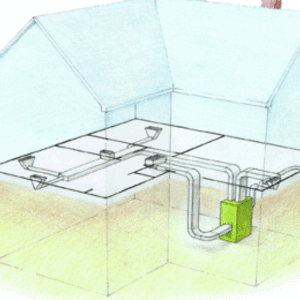
Using regular atmospheric pressure to crush cans of any size is fun, but how does this phenomenon relate to buildings? First, for any air to leak into or out of a building, you need two things: a pathway and a pressure difference. In the photo below, you can see two pathways between the conditioned space below and an unconditioned attic. The pressure could be higher on either side, so you could be getting nasty, hot, humid attic air leaking into your house, or you could be losing your nice warm air to the attic.

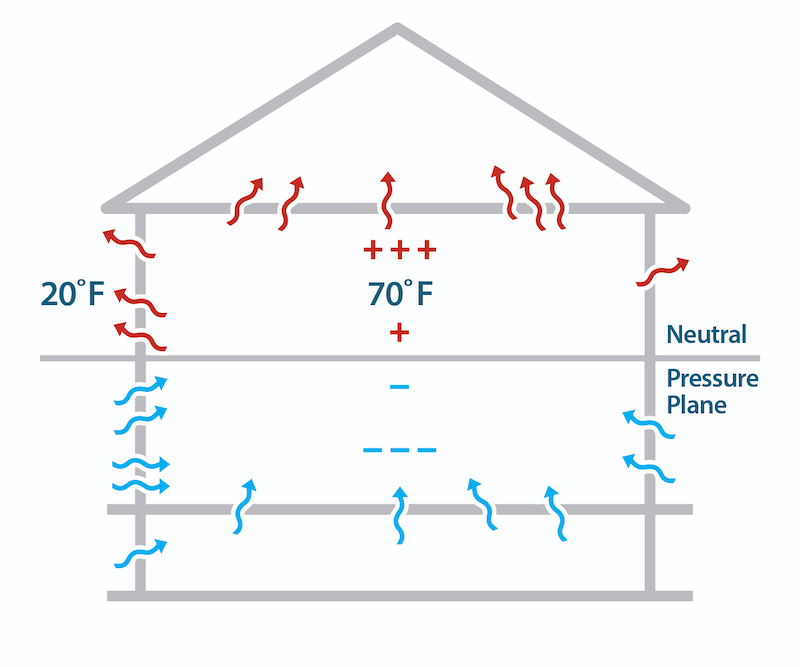
The other aspect of air leakage is the pressure difference. The three main ways they arise across a building enclosure are through the stack effect, wind, or mechanical systems (e.g., exhaust fans). The stack effect is what causes warm air to rise. And that goes back to the weight of all that air sitting on the palm of your hand.
The air pressure on the outside of a building is higher at the bottom than at the top because air pressure decreases with height. The indoor air is usually at a different temperature from outdoors, and that causes the indoor air to be at a different pressure, too. The result is that in winter, air leaks into the house at the bottom and leaks out at the top.
There’s your little beer-can pressure-crunch building-science lesson for the day! (And by the way, Miller Lite isn’t my beer of choice.)
_________________________________________________________________________
Allison A. Bailes III, PhD is a speaker, writer, building science consultant, and the founder of Energy Vanguard in Decatur, Georgia. He has a doctorate in physics and writes the Energy Vanguard Blog. He also has a book on building science coming out in the fall of 2022. You can follow him on Twitter at @EnergyVanguard. Photos courtesy of the author. Lead illustration by Christopher Mills.
Weekly Newsletter
Get building science and energy efficiency advice, plus special offers, in your inbox.













9 Comments
That was fun. I hope people don't now decide that air sealing is dangerous out for fear that atmospheric pressure will crush your house if you boil a kettle for tea and then tightly close your European windows.
A minor suggestion for the nice illustration of stack effect in a house: The blue minus signs below the neutral pressure plane might imply that that region is as cold as the outside air. I think making the minus signs red would be clearer.
Thanks, Charlie. I hope no one takes that away from the article either. Good point on the color of the minus signs. I probably should make all the plus and minus signs black, though, just to separate them from what's going on with temperature.
"First, for any air to leak into or out of a building, you need two things: a pathway and a pressure difference."
They may seem basic or self-evident, but these short building aphorisms are invaluable to keep in your head while designing or building.
I like the thinking 'the way to feel the weight of air is to remove it's pressure from one side.'
The 'solids' analogy would be that if we are holding up a column of iron, we are also resting our hand on a column of iron supported on the floor. Thank god we live in a fluid.
I always think of the shape of air bubbles rising in water (or should I say water falling onto an air bubble) when thinking about the force distribution of a fluid column.
https://www.youtube.com/watch?v=NjB7LXSQoQc
My high school physics teacher always said 'nothing sucks in physics.'
I ran experiments in a column of water where air bubbles would move downward and congregate at the bottom of the column, usually got some inquisitive stares.
A cliff hanger. Well, how'd you do it!?
OK, the secret. Decades ago, when I was young, NASA studied liquid fuel rockets, which would sit and vibrate on the launch pad. The cylindrical liquid fuel tanks would have a headspace where gas in the headspace would be entrained and then move downwardly due to the vibrations. The problem, the outlet to the tank was at the bottom, gas instead of fuel, not good.
I replicated the phenomena using a woofer, a Macintosh (vacuum tube) amplifier and a frequency generator with a plexiglass column of water mounted in the center of the woofer. At the right frequency/range of frequencies, the surface will breakup and entrain air, bubbles will then move to the bottom. We had hypothesized the unit as a possible new way to aerate a bioreactor where air/oxygen is introduced via frequency control of a vibrating column reactor.
People were curious. Fascinating science.
Very interesting.
That reminds me of the levitating liquid pendulum discussed in this Steve Mould video:
https://www.youtube.com/watch?v=gMAKamGIiMc
and here: https://www.youtube.com/watch?v=bodsuTucSxQ
Not sure if that's the same phenomenon you are describing but I'm guessing it is at least related.
Hi Tyler, thanks for the links, great explanation of the viscous fluid (silicone oil) and air, along with the vibrating pivot point and torque. One video has Benjamin Apffel (though he misspells his last name Apfel). Here's an article from Apffel et al.: https://www.pnas.org/doi/pdf/10.1073/pnas.2111214118 (mentions Bjerknes forces). And, another: https://arxiv.org/ftp/arxiv/papers/2003/2003.04777.pdf ("Floating under a levitating liquid").
"Bjerknes forces are translational forces on bubbles in a sound wave. The phenomenon is a type of acoustic radiation force. Primary Bjerknes forces are caused by an external sound field; secondary Bjerknes forces are between pairs of bubbles in the same sound field. They were first described by Vilhelm Bjerknes in his 1906 Fields of Force."
Here's a reference, Hashimoto, "Violent Liquid Sloshing in Vertically Excited Cylindrical Containers", Experimental Thermal and Fluid Science 1988; 1:159-169
First, the surface breaks up and entrains small bubbles (silicone oil may be too viscous to do this), then the bubbles form a cluster. Fig. 17 shows a bubble cluster, which can move downwardly to the bottom of the cylindrical container. From my experiments (back in the 80s), I would not necessarily call the sloshing "violent", depends on various factors.
"As stated above, the free surface motion becomes very violent at large excitation accelerations, and small air bubbles are entrained into the liquid from the liquid surface. These bubbles will not rise to the liquid surface, because the air bubbles in oscillating pressure fields can be trapped in the liquid by the Bjerknes force [11]."
"For instance, the bubble cluster phenomenon can occur in the fuel system of large liquid-propellant rockets, because the vibratory force is transmitted to the liquid system during flight. And bubble formation can seriously impede the performance of the propellant systems that are required to provide fluid transfer."
Log in or create an account to post a comment.
Sign up Log in