
Image Credit: Image #1: Building America Solution Center
I like to tell people I’m a recovering academic. The truth is, though, that I haven’t left physics behind. That would be impossible since I’ve been making a career in the world of building science. So today I’m going to delve into that subset of building science called building physics as we take a look at the physics of water in porous materials. You’ll also learn about the fourth state of water, the one that’s not liquid, not solid, and not vapor.
But fear not! I’m going to do all this with a lot of images, and I won’t include a single equation (although there’s a link to one if you’re brave enough to look).
The polar nature of the water molecule
Let’s begin with the water molecule. One oxygen, two hydrogens, bonded together covalently. That means each hydrogen shares its one electron with the oxygen atom and both atoms get to complete their outer shells by doing so.
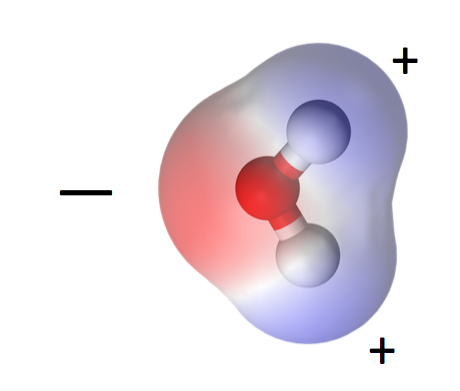
One consequence of water’s polarity is that it is a liquid at higher temperatures than similar molecules. Carbon dioxide, for example, is a linear, nonpolar molecule and becomes a gas at about -71° F (-57° C). If water were like carbon dioxide, the oceans would not be here. Mountains would have no snow. And I wouldn’t be writing this because we humans would not exist, at least not in the form we have now.
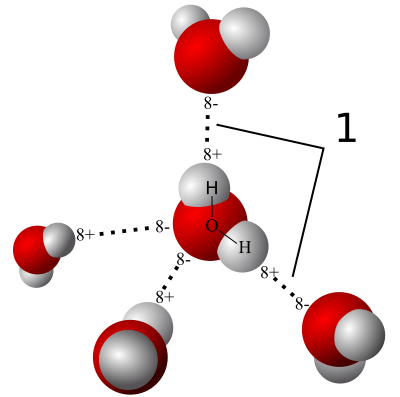
The polarity of water molecules means that when you put it in contact with another material, what happens depends on which attraction is stronger: water for itself or water for the other material.
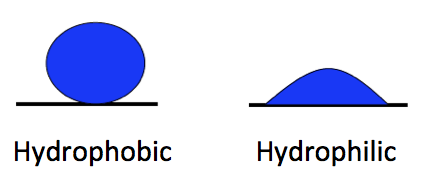
When the water is more strongly attracted to itself, as you see on the left side of the diagram at left, we call the other material hydrophobic, or water fearing. When the water is more strongly attracted to the other material than to itself, we say that material is hydrophilic, or water-loving.
When the source is liquid water
Now it’s time to recall the Second Law of Thermodynamics: Water moves from wet to dry areas.
The photo at the top of the article illustrates perfectly how water can move from the ground into the porous concrete and concrete block. It’s not uncommon for moisture problems in attics to originate with a wet foundation.
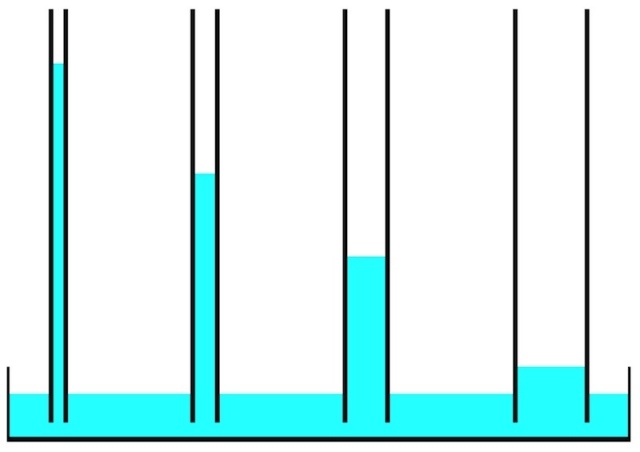
The photo of bricks below shows the capillary rise of water over time. After a bit more than an hour, the water is three-quarters of the way up the brick.

Since trees move moisture from the ground to the leaves using capillary action, the photo below might give you an idea of how high water can rise.

(If you look closely, you might see me standing at the base of the tree. And if you do see me there, I’ll congratulate you on your imagination.)
When the source is water vapor
Liquid is easy. Things start getting fun when we take a look at what happens when water vapor interacts with porous materials. If you have such a material (drywall, wood, concrete, cellulose…) with humid air on one side, water vapor will find its way into the pores. If the material is hydrophilic, water vapor will start sticking to it.
And then we start using a new word. When the surface pulls water out of the air like this, we call it hygroscopic. The material is hygroscopic, and we also say that it has hygroscopic water on the surface.
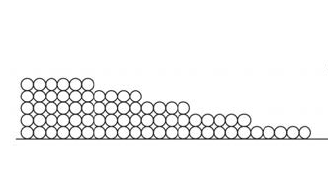
When that first monolayer of water molecules begins sticking to the surface, it does so with gusto. Remember, we’re talking about hygroscopic materials that can pull water vapor from the surrounding space. They really like each other!
The second monolayer is also attracted strongly…but not as strongly as the first monolayer. Since the second monolayer is attracted to the surface through the monolayer of water that’s already there, the attraction is muted a bit. Likewise with the third, fourth, and fifth monolayers.
That attraction can be put in terms of energy. Recall that the energy absorbed or emitted when water boils or condenses is called the latent heat of vaporization. When water vapor adsorbs onto a surface (or breaks free of that adsorption), there’s a latent heat of adsorption involved.
According to Professor Chris Timusk, the heat of adsorption for the first monolayer is 3700 kJ/kg. For the second, it’s 2972 kJ/kg. At the fifth monolayer, the heat of adsorption is 2500 kJ/kg, which is equal to the heat of vaporization for liquid water.
What that means is that the adsorbed water really is different from the other three states. It’s obviously not vapor. It’s not ice either. It’s most similar to liquid water, but it’s not as free to move as liquid because it’s bound more strongly to the surface than it is to the surrounding water molecules. Only when you get more than five monolayers do you see it beginning to act like liquid water.
The three transport modes
Now we’re ready to talk about how water moves through porous materials. The three transport modes are:
Vapor diffusion carries water vapor through the material in the vapor state. It doesn’t stick to any of the surfaces it encounters. It just floats on through in the empty space of the pores. Not much water gets through like that.
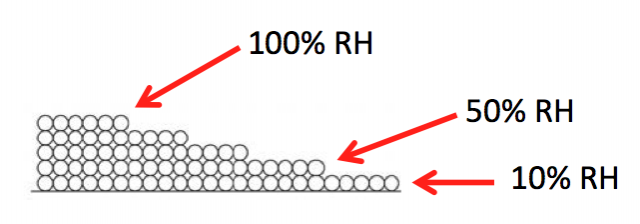
Surface diffusion moves more water than vapor diffusion. This happens because of that attraction I discussed earlier. Since the first monolayer is attracted the most strongly, it’s energetically favorable for a molecule in the second monolayer to move down to the first layer if it can, as shown below.
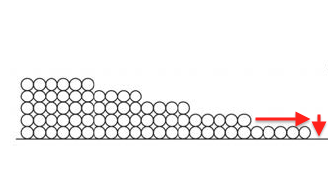
But things really open up when the capillaries start filling up. Once a pore is completely full, water can move more rapidly through the porous material. That’s capillary flow.
Sorption isotherms
Now we can put all this together and understand what’s going on in these geeky things called sorption isotherms. The graph below, taken from Professor Timusk’s doctoral thesis, is a good example.
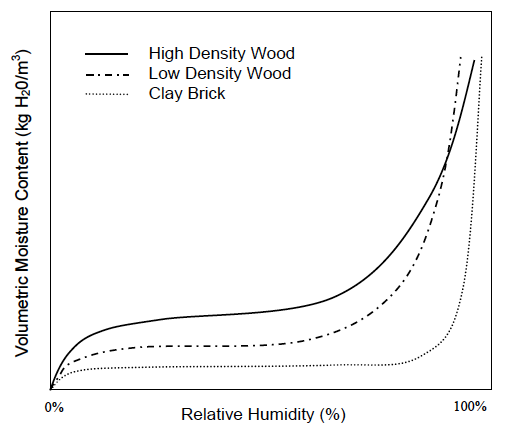
Notice that each curve exhibits the same pattern: a rapid rise, a flattening out, and then another rapid rise. Recalling the explanations above for adsorption and the three types of moisture transport, what do you think is happening in those regions? See if you can figure it out before going on to the next paragraph.

Well, first off, the curves show surface diffusion and capillary flow. We know capillary flow starts at higher relative humidities, so the initial rise and the flattened out part are where surface diffusion is happening. But why does the initial rise flatten out so quickly?
Recall that the first monolayer feels the strongest attraction for the surface. It gets filled at about 10% relative humidity. The flat part of the curve is mostly filling the second monolayer plus a bit more in wood and significantly more in brick.
When the curves shoot up again, capillary flow has kicked in (see graph below). Now the moisture content can increase rapidly with increasing relative humidity. And here’s where we can see something really interesting about the difference between materials.
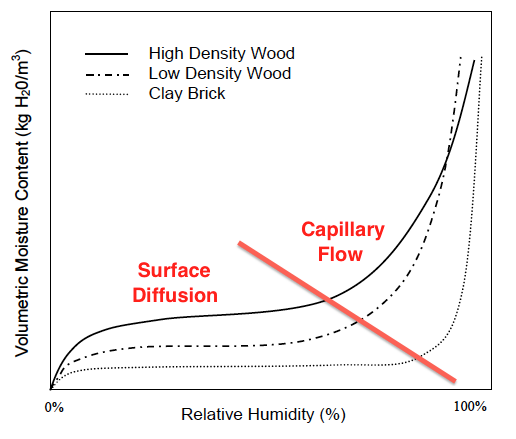
Another important thing to know about sorption isotherms is that the curves you see above are for a specific temperature. As you raise or lower the temperature, the curves shift. Why? Because the amount of moisture a material can hold at a give relative humidity depends on how warm or cool it is.
Warmer materials cannot hold as much moisture because there’s enough heat there to dry them out. Cooler materials hold more moisture. In fact, Bill Rose calls this the Fundamental Rule of Material Wetness: Warm materials tend to be dryer and cool materials tend to be wetter.
And now you can look at sorption isotherms and understand what they’re telling you about materials… without being a scientist or academic, practicing or in recovery.
Sources
The main source I used for this article was chapter 3 of Professor Chris Timusk’s doctoral thesis (pdf). Most of the rest came from Dr. Joseph Lstiburek’s presentations on building science fundamentals and hygrothermal analysis, Wikipedia, and Bill Rose’s book, Water in Buildings.
Allison Bailes of Decatur, Georgia, is a speaker, writer, energy consultant, RESNET-certified trainer, and the author of the Energy Vanguard Blog. Check out his in-depth course, Mastering Building Science at Heatspring Learning Institute, and follow him on Twitter at @EnergyVanguard.
Weekly Newsletter
Get building science and energy efficiency advice, plus special offers, in your inbox.














4 Comments
Thank you!
Dr. B,
That was wonderful. Now some questions... does the capillary flow of concrete increase as the MPa increases? Is there a relationship?
What about Critical Degree of Saturation (Scrit) in concrete: is there a relationship to MPa?
Finally, were the aliens in question using capillary flow or good old fashioned depressurisation to pull up the beer?
Thanks for digging into this, now I can sleep at night.
trees?
There's a problem with using tree sap in explaining capillary flow. The capillaries in building materials all have a meniscus (They have menisci?), or an interface between liquid and vapor. There's no meniscus in a tree. An old guy complimented me once on a tight woodworking joint saying "Looked like it growed that way." Same explanation for water filling the xylem cores. Growed that way.
More on sap
Allison and Bill,
Back in January 2004, I wrote an article on capillarity, noting, "Contrary to popular belief, capillarity is not responsible for the rise of maple sap from underground roots into sap buckets."
A Cornell University web site provides more information on this topic:
"What causes the sap of maple trees to flow in the spring? During warm periods when temperatures rise above freezing, pressure (also called positive pressure) develops in the tree. This pressure causes the sap to flow out of the tree through a wound or tap hole. During cooler periods when temperatures fall below freezing, suction (also called negative pressure) develops, drawing water into the tree through the roots. This replenishes the sap in the tree, allowing it to flow again during the next warm period. Although sap generally flows during the day when temperatures are warm, it has been known to flow at night if temperatures remain above freezing.
"Thus, pressure and suction are essential to sap flow. But how do the pressure and suction develop?
"Sap flows through a portion of the outer tree trunk called sapwood. Sapwood consists of actively growing cells that conduct water and nutrients (sap) from the roots to the branches of the tree. During the day, activity in the cells of sapwood produces carbon dioxide. This carbon dioxide is released to the intercellular spaces in the sapwood. In addition, carbon dioxide in sap is released into the spaces between the cells. Both of these sources of carbon dioxide cause pressure to build up in the cells. A third source of pressure is called osmotic pressure, which is caused by the presence of sugar and other substances dissolved in the sap. When the tree is wounded, as when it is tapped by a maple producer, the pressure forces the sap out of the tree.
"At night or during other times when temperatures go below freezing, the carbon dioxide cools and therefore contracts. Some of the carbon dioxide also becomes dissolved in the cooled sap. Finally, some of the sap freezes. All three of these factors create suction in the tree. This causes water from the soil to be drawn up into the roots and travel up through the sapwood. When temperatures rise above freezing the next day, sap flow begins again."
Solids vs. air
It is interesting to me too, that while solids hold more water when cool, that with air, it's just the opposite. Warmer air holds more moisture than cool air. Interesting world we live in.
Log in or create an account to post a comment.
Sign up Log in