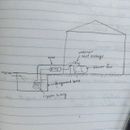
Underground Water Tank for Indoor Cooling
In in my city (developing country, hot humid climate), the city turns on the water mains for a short time daily and it fills an under ground tank (6′ x 6′ x 8′ approx.), constructed, I believe of masonry. We then turn on a pump to pump it to the water cistern on the roof which is then used in the house when you turn on the tap.
I’m wondering if the underground tank would be a good source of space air conditioning using a water-water heat exchanger, an air-water heat exchanger, and a circulatory pump, in a concept similar to the “well-water heat exchanger”, except this is closed-loop.
The idea is that the mains water and the tank are a large reservoir of coolth at earth temperature.
Would the idea work? Does it already exist, and if so, what is it called?
Thank you.
GBA Detail Library
A collection of one thousand construction details organized by climate and house part









Replies
This is similar in concept to what is commonly known as an “ice chiller”. The idea with an ice chiller is to freeze a bunch of water to ice at night when electric rates are low (off-peak time), and then thaw the ice to use it for cooling during the day when electric rates are higher. This allows the big compressor in the chiller to run only during off peak times to save money.
The problem you have is you won’t have a phase change (ice to water), so you have much less available cooling capacity compared to an ice chiller. You have something closer to a geothermal ground-source heat pump system. Cooling capacity is going to be limited to what the ground can sink.
If you’re plan is to just circulate the water from the underground tank through a radiator coil to use it to cool air directly (no compressor or refrigerant involved), you can work out how much cooling capacity you have using the specific heat of water. Basically you can work out, based on the mass of water and the specific heat of water, how much air you can cool before you’ve raised the temperature of the water 10 degrees (for example). I’d run that calculation before building this to determine if you’ll get enough cooling capacity to be useful to you.
Note that if this tank is storing potable (drinking) water, make sure everything you use in your cooling loop is safe for drinking water. Note also that if your cooling system raises the temperature of the water in the tank too much, you get to a point where it is much less likely stuff will start growing in that water. This is another potential health risk with the system you’re considering.
Bill
Thanks Bill. Yes, it is intended to be a geothermal cooling system.
To clarify, the water in the tank is potable, but I'm not pumping that water through the cooling system. The circulatory pump pumps a separate closed loop of water through water-tight* heat exchangers.
*Any leaks that contaminate the potable water in the tank is a concern though.
Another difference with the ice chiller is that I'm not relying on evaporative cooling (which would raise humidity in an already humid environment).
As far as raising the temperature of the underground water tank, the idea is that the conductive material of the tank's walls and large surface area would sink the heat to the surrounding soil and keep the water cool. But I'm not sure how efficiently the heat from the copper tubing would sink to the tank walls and surrounding soil.
Running calculations is good advice. I'm not sure right now how to quantify all the variables, but I can try.
But if anyone has tried something similar, or can identify issues (as you've done, thanks!), or can dismiss it outright as a no-go that would be great.
Ice chillers don't have to dump their heat to an evaporative cooling tower, they can put it anywhere. I had one customer that had a cooling pond with a fountain, for example.
Your 6x6x8 foot tank is 288 cubic feet. At about 7.48 gallons per cubic foot, that's about 2,154 gallons of water. Let's convert that over to metric units to make the rest of the calculations easier: 2,154 gallons is about 8,154 liters.
The specific heat of water is 4,200 joules per kilogram per degree celsius. What this means is it takes 4,200 joules of energy to raise the temperature of one kilogram of water one degree Celsius. Since one liter of water weighs one kilogram (told you metric units would be easier :-), that means it takes 34,246,800 joules of energy to raise your underground tank of water one degree Celsius. We're neglecting the ability of the earth to sink some of that energy, so in your particular case you can actually dump a little MORE energy into the tank before it will rise that one degree C, but how much MORE is difficult to know without testing your particular system.
One joule is one watt*second, So it will take 34,246.8 kilowatt seconds to raise your tank one degree C. That's a goofy unit, but it's equivalent to about 9.5 kilowatt hours since there are 3,600 seconds in one hour. Now we know the water in your tank will rise 1 degree C for each 9.5 kilowatt hours worth of heat you put into it. For a 10 degree C rise like I originally mentioned, that means your tank will sink 95 kwh of energy.
1 kwh is equivalent to 3,412 BTU. That works out to 324,140 BTU to raise your tank 10 degrees C.
That was the easy part, and it's a bit simplified. To work out how much practical cooling you actually have, you need to know the thermal resistance of your radiator, which is a measure of essentially how efficient it is at cooling air with the cool water running through it, and the temperature of the air coming into that radiator. You need to know your starting water temperature too. From all that info, and keeping in mind that as you're cooling the air, you're also raising the temperature of the water (which in turn reduces your ability to cool the air), you can work out how much air can be cooled how much for how long.
The calculations are pretty easy, but you have to be careful to be consistent with your units, especially with units that have a time component (like kilowatt hours) and units that do not (like kilowatts). The first is a quantity of energy, the second is a rate of energy, and they're very different things.
Bill
Thanks very much for this detailed answer and numbers.
This inspired me to write a short script in Matlab to simulate the performance of this system.
I started with a product I found online:
https://chillxchillers.com/air-to-water-heat-exchangers/zephaire-2-speed-air-to-water-heat-exchangers?sku=ZAU0120500ZA-2
It claims 12,500 BTU/hr with a 30F delta-T.
Assuming this fits the equation BTU/hr = x (delta-T), this gives me x = 417 BTU/(hr F).
Then assuming a heat transmission coefficient U=0.4 BTU/(hr ft3 F) for both the room and tank walls, and starting with constant outside tempertaure=95F and constant ground temperature of 70F (which are also the initial temperatures of room air and tank water respectively), I timestep every minute for 5 hours and get an inside temperature settling to 81F after 20 minutes. Water temperature goes up 1.25F in the 5 hours. I've attached the plot as an image.
I'm fairly new at this so I'm not sure if I made any mistakes in the calculations. My code is below in case anyone is interested:
U = 0.4 % Heat trans coeff for walls, BTU/(hr ft2 F)
% Room dimensions
room_dim_l = 20; % ft
room_dim_w = 20; % ft
room_dim_h = 10; % ft
Tg = 70; % Ground temp, F
To = 95; % Outside ambient temp, F
spec_hum = 0.033 % lb/lb
c_s_air = 0.24 + 0.45 * spec_hum; % BTU/(lb F)
dens_air = 0.075; % lb/ft3
dens_water = 62; % lb/ft3
vol_room = room_dim_h * room_dim_l * room_dim_w; % ft3
tank_dim_l = 8; % ft
tank_dim_w = 6; % ft
tank_dim_h = 6; % ft
vol_tank = tank_dim_h * tank_dim_l * tank_dim_w; % ft3
minutes = 5 * 60;
Tw = zeros(1, minutes);
Ti = zeros(1, minutes);
Tw(1) = Tg; % Initial water temperature
Ti(1) = To; % Initial inside temperature
for i = 1:(minutes-1)
% Coolth gain through AC
Hac = (417 * (Ti(i) - Tw(i)))/60; % BTU/min
% Coolth gain through floor
Hfloor = (room_dim_w * room_dim_l * U * (Ti(i) - Tg)) / 60; % BTU/min
% Coolth loss through walls
Hwalls = (((room_dim_w*room_dim_h)*2 + (room_dim_l*room_dim_h)*2 + (room_dim_w*room_dim_l)) * U * (To - Ti(i)))/60; % BTU/min
Hnet_room = Hac + Hfloor - Hwalls; % BTU/min
delta_T_air = Hnet_room / (c_s_air * dens_air * vol_room);
% new inside temp
Ti(i+1) = Ti(i) - delta_T_air;
% Hac heats up water in tank, but some heat loss through tank walls and floor
% Assume negligible heat gain from tank ceiling because of insulating air layer. (not really negligible!)
Hloss_tank = (((tank_dim_w*tank_dim_h)*2 + (tank_dim_l*tank_dim_h)*2 + (tank_dim_w*tank_dim_l)) * U * (Tw(i) - Tg))/60; % BTU/min
Hnet_tank = Hac - Hloss_tank;
delta_T_water = Hnet_tank / (dens_water * vol_tank);
% new temperature of water
Tw(i+1) = Tw(i) + delta_T_water;
end
hours = [1:minutes]/60;
plot(hours, Ti, '-.k');
hold on
plot(hours, Tw, '--b');
ylim([60 100])
ylabel("Temperature (F)");
xlabel("Hours")
legend("Air", "water");
Congrats on trying to model it - we need more of this.
> constant ground temperature of 70F
The ground will heat up and contribute less cooling.
> Assuming this fits the equation....
Most radiators don't, but I don't know about the one you chose.
Thank you. Definitely, the model could be enhanced. I could try to do that with more data and more study on heat physics. Another invalid assumption I thought was my constant density for air. That should change too as the temperature goes down from the cooling.
>> Assuming this fits the equation....
>Most radiators don't, but I don't know about the one you chose.
It would be nice to find a graph of BTU/hr vs delta-T but I wasn't able to find one with the search terms I was using.
This particular product says 12,500 BTU cooling at 30F delta-T and 50,000 BTU heating at 120F delta-T.
50k/120 = 12.5k/30 = 417
which suggests (using only 2 data points) linearity.
In the US, I would consider *not* using complex soldered copper piping that might leak here, but instead using a long, monolithic coil of PEX pipe, with a homemade frame to space it out. It's much lower thermal conductivity, but you can make it enter and leave the tank above the water-line, it's easier to work with, and it's not that expensive (70USD for 300ft x 1/2") considering the high price of copper pipes.
Project example, a recirculating thermal storage tank to store excess solar power and use it for hot water supply and/or hydronic heating:
Overview: https://www.youtube.com/watch?v=ryJmtItfaXQ
Insulation: https://www.youtube.com/watch?v=bI0T7ZegAPU
PEX Heat exchanger: https://www.youtube.com/watch?v=WwTsWUKr5VE
I'm going to give you three things to think about, though.
1) Condensate - any cooling system in a humid place that creates a large temperature drop has to deal with the fact that hot air holds more water vapor than cold air; When air is cooled down significantly (how much depends on your RH), to below its dew point, that water condenses into liquid. In a lot of different situations, if that water isn't collected well and promptly drained, this creates a mold issue.
2) Water sanitation - In the the US, municipal water supplies add chlorine, chloramine, and other chemicals to prevent the water from growing bacteria inside the distribution system. There are places in the US with significantly warmer and significantly cooler ground temperatures. With warm ground temperatures, they add higher levels and different types of chemicals. I haven't calculated what your temperature drop would be in your situation, but I can imagine a situation where you raise the storage water temperature to levels that create a sanitation issue. Maybe it's a negligible concern, I don't know.
3) Airtightness - This is the kind of system that can make a lot of sense in a very well-insulated, tightly sealed house which needs barely any space heating/cooling in the first place. In a house where two people have windows open in their bedroom, or where the rafters aren't sealed, or where the walls are uninsulated, it might take an HVAC system 10 times as powerful.
Thank you. All good points. Except I don't know if I have to worry about condensate given the steady-state temperature seems above the dew point in my use case scenario.
For a worst-case scenario with condensate, just take your entering water temperature at the start of a run, which is the lowest temperature your system will see, and see if that is below the dew point of your air. If that lowest starting temperature isn’t below the dew point, you won’t get any condensation. My guess is your condensation issues will be minimal compared to what you might see with an AC setup since you’re dealing with much lower delta T values.
Nice job with the modeling, BTW. I can’t offer much insight into matlab stuff since I haven’t used that system at all since college over 20 year ago.
In regards to the comments about using PEX instead of copper for a heat exchanger, I would suggest using soft copper tubing instead of rigid copper stick pipe. The soft copper tubing can be hand formed, and will let you put in more pipe with fewer connections. If you want to make super secure soldering connections, you can either braze them, or use silver solder. Either will make a better joint than regular solder.
Bill
My gut tells me this isn't going to work. I think the ground cooling capacity is going to be exhausted in very short order, like a matter of a few days. That's assuming that the tank can even transfer the heat to the soil quickly enough to be useful, which is another big question.
I have a similar system under my house, except instead of a tank of water I have pipes buried directly in the ground. In the summer this is used to cool the incoming ventilation air. Compared to your situation, all the conditions are in my favour. I am in a temperate climate, so the ground temperate is much lower than yours (about 10C or 50F). The incoming air I'm cooling is probably not much different than the air you''re trying to cool, certainly not higher (25-30C or 77-86F). The amount of air I'm cooling is definitely smaller than the amount you're trying to cool. My system has contact with way more earth than your water tank. Even with all of that, the temperature of the water in the system noticeably increases throughout the summer and even over short periods of higher demand (longer run times with higher outdoor temperature).
I'm not sure there's an accurate way to actually model this kind of thing at present. There's a lot of variables that are changing over time and are dependent on other changing variables.
If you do decide to go ahead with this, I can't think of a reason why you'd need to separate the potable water from the water in the loop. Just make sure all the materials are rated for potable water use. Then you don't have to worry about leaks and contamination. It would likely be more efficient if you're just pulling the cool water from the tank and returning warm water, rather than using a heat exchanger. You could draw the cool water from the bottom of the tank and return the warm to the top; stratification would tend to keep the warmer water at the top.
Would be interesting to model the effect of blowing a little air above the tank to encourage evaporative cooling. Also interesting to compare to PV solar panels attached to a solar powered air conditioner (expensive but effective).
One problem with your setup is that for you to continue to cool day in day out, you also have to get that water back to 70F. The tank doesn't have enough surface area to transfer the heat so that means using up enough water to bring the temprature back down. Don't know how the cost of that compares with AC costs. The cooling is also not quite free, you still have to run a pump and a blower.
The bigger issue with this setup is that you are only dealing with sensible cooling, your ground water is just not cold enough for much de-humidification.
In hot humid climate most of your cooling load is moisture removal, which this setup will do very little. It also will significantly increase the interior RH. For example, if you are cooling 95F air 60%RH to 80F it will be 100% RH. Not the most comfortable room to be in, if anything it is less comfortable than the 95F room. Never mind mold issues.
To deal with the RH you'll have to run an AC unit anyways, might as well use that for the actual cooling.
All very good points. Thanks everyone.
I see it’s probably not worth building, even as an experiment, unless I somehow was able to get some free salvageable parts.
One note that hasn't been discussed here - the tank gets refilled from the mains. This brings new, cool water into the system, essentially making it into an open system, with the warmer water being pumped to the roof cistern for use, and cooler water at ground temperature replacing it. That will improve system performance, at whatever the water usage rate for the house is.
I havebeen thinking similar idea to have passive cooling for th house, the difference is id put the tank right under the house and let the floor do the heat exchange.
Another idea s to put piping system on air inlet ventilation. Intake is on ground level, outlet on top of the room as hot air will rise.
The aim is to average out nighttime temp with daytime hence reduced electricity for ac.
Anotther idea is to cover roof with water absorping fabrics such as cotton and wet them and let the evaporationcool the house.
I might try small scale experiment tho to be sure.
Heat exchange in the underground tank would be efficient if the coils follow something like the attached image, since cold water always remain at the bottom and warm water rises.
You don't need a coil, just draw off the tank directly. If you want to use the tank for cooling, you would extract cold water from near the bottom of the tank, and recirculate the slightly warmer water into the top of the tank. If you want to use the tank for heating, you'd do the opposite. This is "countercurrent" circulation, which makes stratification in the tank work for you instead of against you. With your prosed flat coil, you won't be able to fully utilize the thermal capacity of the entire tank. Water heaters work similarly in terms of where the inject and draw off water.
The bigger issue is the cost effectiveness of this system, and it probably won't be very cost effective. Even ice chillers, which use phase change (you get a LOT more energy involved whenever you change from solid to liquid, or from liquid to vapor, or vice versa) aren't very cost effective in many areas anymore due to the on and off peak electrical rates getting too close to each other. With a passive system, you will have far less thermal storage capacity, and much reduced transfer efficiency due to the much smaller thermal gradients involved.
If you want to economize on cooling, I would put in a small cooling pond and circulate the water through a heat exchanger at the condenser (the outdoor unit) of your air conditioner. The cooling pond would allow for some evaporative cooling, but it's main function would be to act as a big heat sink to help keep the effective temperature of the air conditioner's condenser much lower than it would otherwise be, which will allow the compressor to see less load, which will mean the compressor will need less energy to do it's job. A system like this will be more economical to install than a full passive system, and will be useful over a wider range of envionrmental conditions.
Bill
Hi everyone,
I'm fascinated by this thread. I've become obsessed with air conditioning living in Florida, and now my bills are going up almost double what they were a few years ago.
I'm currently running a split unit that's powered by off-grid solar. It's not novel, but it's very rewarding when the sun's beating on your house and you're getting free air.
I have a question for you, Bill. One interesting thing that I discovered is that my house was built in the 30s and has an elevated crawl space of about 18". When I placed the split unit next to the side of the house, I noticed that cool air was being pulled into the outdoor condenser from underneath the house. This was a pleasant surprise, so I decided to test it out by moving the line set away from the cool air. I saw an approximate 5-10% increase in load and temperature at the hotter non-cooler air input.
My question is, has there been any research done about strictly cooling the air going into the outdoor split unit? I'm not talking about burying the line sets, but literally cooling the air that's being pushed past the condenser. I'm curious to know your thoughts on this. Basically, running a large air duct under the ground to assist air around the split?
Thanks in advance!
The penalty of running the duct outside the conditioned space would exceed the efficiency gain of pre-cooling the indoor air. You will lose too much “coolness” to the outdoors (or rather, gain too much heat from the outdoors). Your setup works now because the cooler crawlspace air is free. Find a way to make air near the condenser cooler for free or low cost and it might work. Maybe a swamp cooler style water feature that relies only on city water pressure instead of a pump?
You don't need to do any new research, the effects are already known. If you are using cooler air in the outdoor condenser, the thermal difference (delta T) between the indoor out outdoor temperature is less, which means less work for the system to pump heat against, so less input energy for the same amount of cooling capacity in BTU. It's no different than your unit having an easier time cooling your home on a cooler day compared to a hotter day.
The issue is where are you going to get that cooler air? Under your home is cool because it's "lost" cold from your home (i.e. some cold air leaks out under the home), and it's probably relatively static -- so it just sits there "being" cool. If you draw from that reserve of cool air, it's like a battery, and eventually the warm outdoor air that gets drawn under your home to replace the cool air you extract will reach an equilibrium and you'll end up back where you started. Think of this as draining a battery -- once the battery is dead, there is no more capacity. You get a TEMPORARY increase in efficiency.
Evaporative cooling is another way to reduce the load on the outdoor unit, but it won't work well in a humid area like much of Florida. Your best bet would be to use ocean water or a lake as a "reservoir of cold", and in those cases, the reservoir tends to be so large that you don't really see it warm up as you use it.
There is no easy way to get around the laws of physics, unfortunately. Moving a certain amount of heat across a temperature gradient requires a certain amount of energy. The easiest way for you to cut down on your operating costs in a region that runs a lot of A/C time over a year is to get the highest SEER rating you can find on your air conditioning system.
Bill
What if…. The water comes from a well… 400’ deep and can produce 15GPM, I put a thermostat to pump the water out if it gets too warm. Put a drain line at the top of the tank that simply dumps the water back into the well.
The tank would be 2500 gallon Polly plastic and the PEX Pipe would be 3/4”
A 5 ton coil and a 2000 CFM fan blowing on the coil…..
this is what I had imagined when falling upon this thread…. Simple condensation pan with a pump to empty the pan…
15 GPM through 3/4" pex is a pressure drop of 424 feet of head. Add that to the 400' of well and some more pressure drop to move the water around once you get to the surface and you're probably looking at 900' total head. Setting aside the practical concerns of operating at that pressure, it would take 5.7 HP to pump 15 GPM at that pressure, or 4,240 Watts.
Assuming you get the full five tons, 60,000 BTU/hr, that's an EER of 14.1 and a COP of 4.2. You can do better than that with an air conditioner.
Hi Bill and Everyone else (:
I noticed something interesting in all those geothermal videos! They use PEX tubing, but isn't that plastic? Plastic feels like an insulator, so wouldn't metal be better for transferring the cool liquid? Copper seems ideal, but I know it's expensive. Still, for best efficiency, shouldn't they use a mix of materials? Have there been studies on the impact of pipe materials in geothermal systems?
Yes, it's plastic and a better insulator than metal. I don't know that there's been studies, but someone has certainly done the math and figured out that using copper doesn't justify the extra expense. It's also more prone to failure.
cool_air,
Burying rigid copper would be asking for leaks.