EPS Foam and Moisture Retention
I use a lot of EPS foam below grade, and never worry about it taking on moisture because it is closed-cell. One thing puzzles me though.
I used to do a lot of work for a resort with 25 hot tubs. Over time the EPS foam in their lids would take on water until they were too heavy to lift. What’s different about the conditions that cause it to retain water, and is this ever a concern in other circumstances?
GBA Detail Library
A collection of one thousand construction details organized by climate and house part






Replies
As I understand it, the issue with moisture retention with EPS is that while the cells in the material are closed, the spaces around them are not. EPS is made by Expanding PolyStyrene (the "E" and "PS" in the name) using steam, but the polystyrene starts out as a bunch of beads. If you look at at a piece of EPS, especially the low density stuff, you can see that it looks like a bunch of beads stuck together. The water can apparently get in between the expanded beads and get into the matrix of the material that way. XPS isn't made the same way, and doesn't have that issue. That is the one (and probably only) advantage to XPS.
The study I saw about this was done I believe by the Alaskan highway commission (I might not have that name right), that apparently insulates under some sections of roadway. They found that over time, and I think it was a 20 year period they tested, EPS took on more water than XPS under similar conditions. That's the one, and only, reason I usually recommend XPS for underground applications where the insulation will be continually exposed to high levels of moisture.
All that fun stuff said, I think EPS is absolutely fine in areas where it is NOT *continually* exposed to moisture. This means anywhere above ground, and in areas where it might see *ocassional* moisture, but not *coninuous* moisture, and by "moisture" here I mean liquid water, not water vapor. EPS is better about water retention than polyiso too, so it's good for things like basements and crawlspaces that have ocassional bulk water problems.
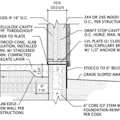
If you want to use EPS underground, such as under a slab or on the exterior of foundation wall, I would provide a bit of protection so that it can drain and not potentially be held in contact with bulk water for extended periods of time. Under slab, I would put a few inch layer of clean stone as a capillary break and a sort of thin, planar drywell, then the EPS on top of that, and the slab on top of the EPS. This ensures the EPS is unlikely to be in contact with water for extended periods of time unless you have severe water problems, in which case you probably have bigger issues than water logged insulation.
On the exterior of a foundation wall, a layer of dimple mat between the soil and the EPS, and the EPS directly against the foundation provides for a sort of "underground rain screen", and a drainage plane. Try to tie the bottom of the dimple mat into the gravel around any perimeter drains to ensure that there is a good drainage path to carry away any water that might get into the immediate area of the EPS layer.
Note that both of my proposed protective measures for underground EPS also have other advantages, so it's not overkill just there to protect a layer of insulation that is usually considered to be moisture tolerant.
Bill
I have no idea, but I wonder if the high vapor pressure is at play. EPS has some vapor permeability, and I would bet that vapor molecules more readily enter the eps than liquid water. With a hit tub (very warm and humid on one side, cold on the other) there is significant vapor drive. And then what is the eps wrapped in? Is that impermeable? If so, I could see how vapor could enter the foam faster than it can exit out the top cold, impermeable layer.
maine_tyler,
The EPS pieces are (imperfectly) wrapped in poly and zipped into the vinyl cover. When you take the heavy sodden ones out, you can dry them in a warm room, but it takes weeks.
I don't think it's a problem that would affect anything we do as builders, but it's always puzzled me.
Malcolm, I've experienced the same thing, and think that it's a result of having 105° water on one side and cold air on the other side, so vapor drive is strong and in one direction, fully saturating the foam. I also wonder if chemicals such as chlorine act as a surfactant, allowing more moisture to penetrate the EPS pores. I also wonder why they don't use XPS for the covers instead. I often rail against XPS on GBA but it is more resistant to moisture accumulation than EPS.
Lobster bouys are made of EPS and seem to handle sodium chloride-laden water just fine.
Have you measured the amount of water in those covers, or otherwise absorbed by EPS? I wonder if it exceeds the amount listed as a maximum in ASTM C518. I'll attach a screen shot below. I'd estimate that it's about the maximum listed for water absorption. I think they probably use Type 1 foam; if it's 3" x 90" x 90", it could absorb up to 4 gallons of water, adding about 30 lbs to the cover.
Michael,
That's an interesting point about the role chlorination may play. I haven't worked at the resort of years now, but as I remember the lids were maybe 20 lbs new but could get so one person could hardly lift them into a pickup truck.
The situation does seem to be unique to the hot tub lids, as EPS is used here for flotation on docks and the like without incident.
Do you know if EPS loses much of its resistance to heat transfer when it is at its maximum water absorption?
Malcolm, I could be wrong but I believe the styrene beads do most of the insulating, with the spaces around the beads either adding a bit more R-value or filling with water which would reduce the R-value. With chemical assistance (such as chlorine) I wonder if some water gets into the styrene beads. In any case, I'm sure being water-logged does not help with R-value.
You may be on to something with the heat of the water vapor. Hot vapor will open up any pores/channels in the EPS material and make it easier for the moisture to get inside. I do know the EPS used in floating bouys does take on some water over time though, but I wouldn't be surprised if the hotter things are, the worse the problem becomes.
BTW, I agree with you that the interior of the expanded beads are probably doing most of the "work" of insulating. Water getting into the spaces around the beads is likely to act like a network of little thermal bridges, reducing the overall R value of the material similar to how studs impact a regular studwall's overall insulating value when insulated with batts.
Bill